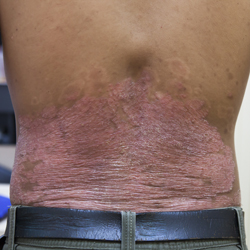
psoriatic arthritis
FDA Grants Second Indication for Skyrizi
Risankizumab-rzaa is an interleukin-23 (IL-23) inhibitor that selectively blocks IL-23 by binding to its p19 ...
JANUARY 24, 2022
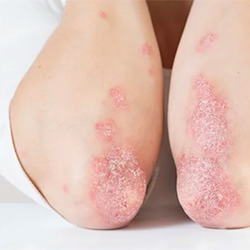
FDA Approves Hadlima Biosimilar for RA, Other Inflammatory Condition
Hadlima was approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic ...
AUGUST 27, 2019
Secukinumab Rx Improves Quality-of-Life Measurements as Early as 4 Weeks
Findings suggest that helping patients feel better through improved quality of life and ability to function ...
JANUARY 28, 2019
Load more
