Octapharma
FDA Grants New Indication for Octagam 10% for Adult Dermatomyositis
The FDA granted a new indication to Octagam 10% IVIG for the treatment of adult dermatomyositis, a rare ...
JULY 21, 2021
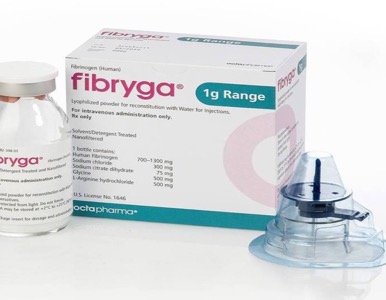
FDA Expands Pediatric Labeling for Fibryga; Updates Research in Label for Octaplas
The FDA approved Octapharma supplements to two Biological License Applications, including an expanded indication ...
MARCH 29, 2021
Octapharma USA Grant Helps Hemophiliac Man Climb the World’s Seven Summits
Octapharma’s support ties into its goal of creatively demonstrating that patients with bleeding disorders, ...
NOVEMBER 1, 2017

FDA Grants Octagam Orphan Drug Designation
The cause of dermatomyositis is unknown. The estimated incidence of dermatomyositis is 9.63 cases per million ...
MAY 17, 2017
