In December 2015, the FDA approved sugammadex (Bridion, Merck), an antidote and the first selective relaxant binding agent available in the United States for reversal of shallow and profound neuromuscular blockade (NMB) induced by rocuronium or vecuronium in adults undergoing surgery. Neuromuscular blocking agents (NMBAs) are essential in general anesthesia; the medications produce skeletal muscle relaxation, which facilitates endotracheal intubation while providing optimal surgical conditions. However, NMBAs have the potential for causing postoperative residual paralysis, requiring the use of a reversal agent.
In 2002, to help improve outcomes in postanesthetic care, the American Society of Anesthesiologists (ASA) released a practice guideline addressing the reversal of residual NMB. The guidelines suggest that neostigmine is effective for reversal of neuromuscular blockers and should be administered when indicated.1 This recommendation was also consistent in the updated, and most current, 2013 Updated Practice Guidelines for Postanesthetic Care from the ASA.2 While the 2002 guidelines from the ASHP and the Society of Critical Care Medicine do not mention neostigmine for reversal of NMBAs, they provide recommendations to monitor the degree of NMB and use the lowest effective dose to minimize the risk for adverse events.3
Neostigmine is an acetylcholinesterase inhibitor that works by decreasing the enzymatic breakdown of acetylcholine, and to a lesser extent, by increasing the release of acetylcholine from the motor nerve terminal.4 This process results in the overall accumulation of acetylcholine, causing displacement of NMBAs from their receptors.4 Although this displacement mechanism does result in reversal of NMB, it is not without limitations. Because a concentration gradient is necessary for NMBA displacement, neostigmine is unable to reverse deeper levels of blockade when high NMBA concentrations are present, resulting in insufficient recovery of neuromuscular function.4,5 Additionally, due to a slow onset time of 20 to 30 minutes, neostigmine is unable to provide immediate NMBA reversal.6
Administration of neostigmine may also cause undesirable effects. Systemic increases of acetylcholine can potentially lead to cardiovascular, respiratory, and gastrointestinal adverse reactions.4 To offset these effects, an anticholinergic agent, such as glycopyrrolate, is coadministered with neostigmine. The addition of this agent may also have undesirable effects, including constipation, blurred vision, tachycardia, and sedation.6,7 As a result of these side effects and the restricted mechanism of action of neostigmine, there was a need for an improved agent with better tolerability.
Sugammadex, a modified gamma cyclodextrin, is composed of cyclic oligosaccharides that form a hollow, doughnut-like structure.5 This structure consists of both a hydrophilic exterior and a hydrophobic cavity, making it capable of forming a water-soluble, guest–host complex with aminosteroid NMBAs in a 1:1 ratio.5,8 This guest–host complex has a very high association rate, and an equally low dissociation rate.5 This results in a very strong bond and rapid removal of NMBAs from the plasma (rocuronium > vecuronium), rendering them incapable of binding to their postsynaptic targets.5 It is estimated that only 1 of 25 million sugammadex–rocuronium complexes dissociates.8 This rapid removal of rocuronium creates a strong concentration gradient and causes remaining rocuronium molecules to diffuse away from the neuromuscular junction into the plasma, where they are quickly bound by free sugammadex molecules, resulting in reversal of the NMB.5
| |||||||||||||||||||||
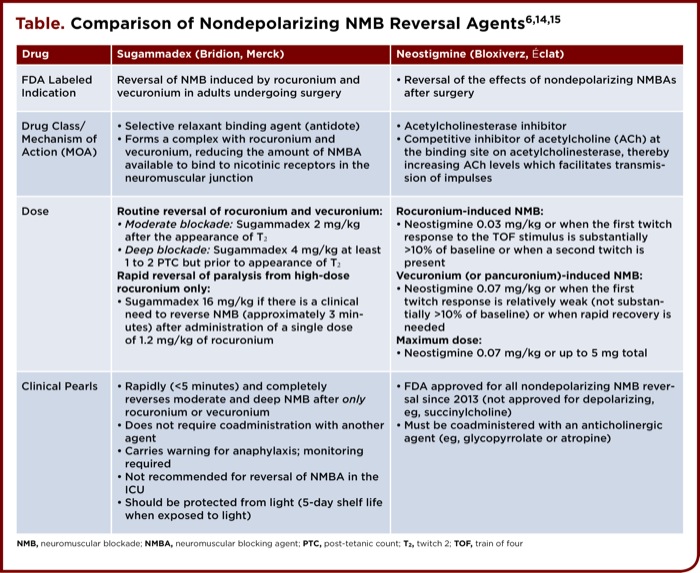
Sugammadex is supplied as a 200 mg/2 mL or 500 mg/5 mL clear solution for IV administration.6 Dosing and other key features of sugammadex and neostigmine are provided in the table. Dosing is based on actual body weight, desired timeframe of reversal, and extent of NMB, which is typically monitored by the train-of-four (TOF) technique.9 This approach applies a series of four stimuli, referred to as “train,” to the patient at a frequency of 4 Hz for 2 seconds, resulting in 4 twitches depending on the level of NMB; 3 of 4 twitches correlate with approximately 75% blockade, 2 of 4 with 80% blockade, 1 of 4 with 90% blockade, and 0 of 4 to almost 100% blockade.9 Generally, it is suggested that optimal blockade is between T1 and T2, or between 80% and 90%.9 To determine the level of blockade when all 4 twitches are present, the TOF ratio is used, which is the ratio of the amplitude of the fourth twitch to the first twitch. It is accepted that a TOF ratio of 0.9 is an indication of adequate NMB reversal and readiness for tracheal extubation, and is therefore used as a primary outcome in many studies assessing NMBA reversal.10 Additionally, in situations of profound NMB with no response to TOF, post-tetanic count can be used.10 This technique is similar to the TOF, but with higher frequency and duration, and helps indicate when the first TOF twitch will reappear.10
Clinical Studies
Several clinical studies have evaluated the efficacy of sugammadex for reversal of moderate and deep blockade. Two similar Phase III trials were conducted that assessed the effects of sugammadex on moderate and deep blockade. Key exclusion criteria were patients expected to have a difficult airway, known or suspected neuromuscular disorders, significant renal dysfunction, or significant hepatic dysfunction. The primary end point in both trials was mean time to recovery to TOF ratio of 0.9. Khuenl-Brady et al11 assessed sugammadex 2 mg/kg (n=46) vs. neostigmine plus glycopyrrolate (n=34) for the reversal of vecuronium in patients under general anesthesia with sevoflurane. Mean time to recovery in patients receiving sugammadex was 2.7 minutes versus 17.9 minutes for neostigmine plus glycopyrrolate (P<0.0001). Jones et al evaluated sugammadex 4 mg/kg (n=37) vs. neostigmine (n=37) for the reversal of rocuronium in patients undergoing elective surgery. Reversal time was 2.9 minutes for sugammadex and 50.4 minutes for neostigmine plus glycopyrrolate (P<0.0001).
Lee et al12 evaluated sugammadex 16 mg/kg (n=55) for rapid rocuronium reversal compared with spontaneous recovery with succinylcholine (n=55). Recovery time was significantly shorter in the sugammadex group than the succinylcholine group: 4.4 versus 7.1 minutes (P<0.001). All three of these studies reported no serious adverse events; the most commonly reported adverse effects were hypotension, pain, headache, and nausea.
Clinical Pearls and Summary
Sugammadex is only effective against the steroidal NMBAs rocuronium and vecuronium due to its inability to encapsulate the other NMBAs, thus excluding reversal of succinylcholine and cisatracurium. Dosing is based on actual body weight, and is not approved for pediatric patients younger than 17 years old, patients in ICUs, and those with severe renal impairment.
Before its 2016 approval, sugammadex was rejected twice, with the FDA citing concerns about potentially dangerous adverse effects and allergic reactions.13 The prescribing information notes that “anaphylaxis has occurred in 0.3% of healthy volunteers.”14 As such, sugammadex carries a warning of anaphylaxis, hypersensitivity, and marked bradycardia that clinicians should be aware of and prepared to manage in the event of their occurrence. Clinicians should monitor patients for an appropriate period after sugammadex is given and be prepared to administer anticholinergic agents (eg, atropine) if needed.
Patients receiving treatment with anticoagulants such as warfarin, unfractionated heparin, low-molecular-weight heparin, rivaroxaban (Xarelto, Janssen), and dabigatran (Pradaxa, Boehringer Ingelheim) should be monitored closely for bleeding, as in vitro studies have shown an increase in activated partial thromboplastin time, prothrombin time, and international normalized ratio prolongations.14 In addition, women using hormonal contraceptives should be advised to use a nonhormonal back-up method for seven days after sugammadex was administered.14
Another consideration for the pharmacy will be how sugammadex is stored and accessed by members of anesthesiology. It is recommended by the manufacturer that the vials be stored at room temperature and protected from light.14 There are 10 clear vials per box and if not protected from light, the shelf life is only 5 days, which may pose challenges when considering stocking in procedural areas. Education on storage concerns may be needed for those involved in ordering, dispensing, and administering. Pharmacy should review with medical staff the need to stock sugammadex in emergency kits.
Results from clinical trials demonstrated that sugammadex significantly reduced time to recovery after dosing with rocuronium and vecuronium, compared with neostigmine plus glycopyrrolate in patients undergoing an elective surgical procedure under general anesthesia. Institutions that choose to add sugammadex to the formulary should first evaluate their NMBA reversal practices and the impact this addition would have on operating and recovery room flow. Specific order sets and protocols should be created to clearly identify which patients and procedures would most likely benefit from the use of sugammadex, that are aligned with current evidence-based medicine. A comparison of sugammadex and neostigmine is provided in the table.
References
- Practice guidelines for postanesthetic care: a report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2002;96(3):742-752.
- Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307.
- Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Am J Health Syst Pharm. 2002;59(2):179-195.
- Jones RK, Caldwell JE, Brull SJ, et al. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109(5):816-824.
- Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104(3):575-581.
- Lexi-Drugs Online. http://online.lexi.com.proxy.pba.edu/lco/action/home. Accessed August 28, 2016.
- Hibbs RE, Zambon AC. Agents acting at the neuromuscular junction and autonomic ganglia. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:266-276. http://tinyurl.com/zp8o7cd. Accessed August 28, 2016.
- Nag K, Singh DR, Shetti AN, et al. Sugammadex: A revolutionary drug in neuromuscular pharmacology. Anesth Essays Res. 2013;7(3):302-306.
- Jurado L, Allison Ta, Gulbis B, et al. Neuromuscular blocking agents. In: Cohen H, ed. Casebook in Clinical Pharmacokinetics and Drug Dosing. New York, NY: McGraw-Hill; 2015. http://accesspharmacy.mhmedical.com/content.aspx?bookid=1514&Sectionid=88804397. Accessed August 28, 2016.
- McGrath CD, Hunter J. Monitoring of neuromuscular block. Continuing Education in Anaesthesia, Critical Care & Pain. 2006;6(1):7.
- Khuenl-Brady KS, Wattwil M, Vanacker BF, et al. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesth Analg. 2010;110(1):64-73.
- Lee C, Jahr JS, Candiotti KA, et al. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: a comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009; 110(5):1020-1025.
- US Food and Drug Administration. Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC). www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/anestheticandanalgesicdrugproductsadvisorycommittee/ucm478975.pdf. Accessed August 28, 2016.
- Bridion [package insert]. Kenilworth, NJ: Merck; December 2015. www.merck.com/product/usa/pi_circulars/b/bridion/bridion_pi.pdf. Accessed August 28, 2016.
- Bloxiverz [package insert]. Chesterfield, MO: éclat Pharmaceuticals; October 2015. Available at: http://bloxiverz.com/data-info/uploads/2016/02/Bloxiverz_0.51mg_Leaflet_Website_FIN.pdf. Accessed August 28, 2016.