The word “same” is on repeat in the FDA’s definition of biosimilars. Compared with the reference product, biosimilars must be given via the same route of administration; have the same strength and dosage form; have the same potential side effects; and provide the same treatment benefits, according to the agency’s latest guidance document (bit.ly/3C4ezPi).
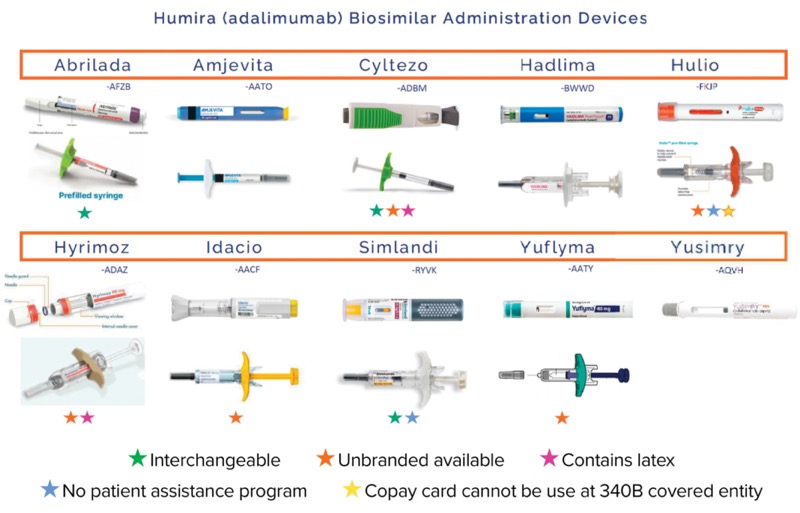
Although those concepts do apply to the 10 biosimilars for adalimumab (Humira, AbbVie) that have been approved and marketed to date—and all share the same indications of the reference product— there are still several factors that differentiate each of these biosimilars from one another, according to Sarah Yelenich, PharmD, clinical services manager at the University of Arkansas for Medical Sciences Specialty Pharmacy, in Little Rock. Those factors include interchangeability, patient assistance/financial support programs, concentration options, formulations containing latex, availability of an unbranded formula, and availability of various dosage forms.
For example, adalimumab-afzb (Abrilada, Pfizer), adalimumab-adbm (Cyltezo, Boehringer Ingelheim) and adalimumab-ryvk (Simlandi, Alvotech/Teva) have been designated interchangeable by the FDA; Simlandi and adalimumab-fkjp (Hulio, Mylan/Viatris/Fujifilm Kyowa Kirin/Biocon) have no patient assistance program, and Hulio’s copay card cannot be used at a 340B covered entity; and Cyltezo and adalimumab-adaz (Hyrimoz, Sandoz/Novartis) contain latex, Dr. Yelenich noted.
“Also, the different auto-injectors may be a factor for some patients,” she said. “If the patient or caregiver has any sort of functional limitations, such as rheumatoid arthritis with hand limitations, one might be more ergonomic than another for dose administration.”
Another key distinguishing factor is financial support. “For commercially insured patients, all the adalimumab biosimilars do have copay cards,” Dr. Yelenich said. “Of course, the big print states ‘Patient copay as little as $0 per fill,’ but that isn’t the whole story.”
Some, she explained, impose maximum benefits per year. “While many do not disclose what that maximum benefit is, Abrilada’s ranges from $4,000 to $14,000 a year, and originator Humira’s is $14,000 a year,” Dr. Yelenich said. “That’s useful information to know if you’re running your patient’s prescription through on their primary coverage and getting an approved claim, because then you can calculate what’s left for secondary billing on the copay card.”
Most Medicare Part D patients who still have a high copay after prior authorization approval are sent to patient assistance programs, as they’re not eligible for utilization of manufacturer copay cards as secondary billing. “For those patients, it’s important to know which biosimilars do not have such programs. That includes Hulio and Simlandi at this time,” she said.
All adalimumab biosimilars (as well as Humira) also have auto-injector misfire replacement programs. “This will be something that you utilize a lot,” Dr. Yelenich said. “If a patient has a misfire with the medication, there is a phone number available from the manufacturer. Ensure that they have the lot number and expiration from their box; if they’ve already discarded their box, this information should be documented in your pharmacy software system, especially if you are an accredited specialty pharmacy.”
The patient must then call to report the misfire themselves, she said; most manufacturers will not allow the pharmacy or the physician’s office to do that.
One-Size-Fits-All Won’t Work
“With current formulary coverage, it’s hard to find one adalimumab biosimilar product that fits your entire basket of patients,” Jon Martin, the U.S. commercial lead for biosimilars at Organon, which commercializes adalimumab biosimilar adalimumab-bwwd (Hadlima), developed and manufactured by Samsung Bioepis, told Specialty Pharmacy Continuum. “When you are onboarding multiple products, you must consider all these different factors: There’s the economic element and the value of a low WAC [wholesale acquisition cost] product.” Additionally, “there’s a supply chain element and confidence in utilization,” Mr. Martin said. “People are also often looking for commonality, a presentation that is very similar to the reference product.”
Finally, he said, “you also have to decide what the right number of biosimilars is for your formulary. Is it two, three or more?”
None of the individual adalimumab biosimilars appears to have significantly outpaced any of the others in terms of uptake, noted Javon Artis, PharmD, the specialty pharmacy director at Visante. At some point in 2025, “we will probably have more information on which biosimilars have broken through with greater adoption,” Dr. Artis said. Right now, however, “we’re at the crux of implementation, and we just need to wait and see which ones will be more widely utilized.”
The sources reported no relevant financial disclosures.
This article is from the March 2025 print issue.