Originally published by our sister publication Infectious Disease Special Edition
By IDSE News Staff
The FDA has approved the second monoclonal antibody (mAb) for the prevention of respiratory syncytial virus (RSV) in neonates and infants, clesrovimab-cfor (Enflonsia, Merck).
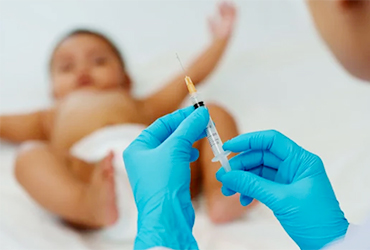
Clesrovimab is indicated for all newborns and infants who are born during or entering their first RSV season. The injection is only one dose of 105 mg, no matter what the infant’s weight, compared with the earlier
